Electron
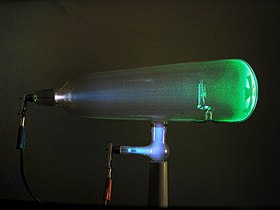 Experiments with a Crookes tube first demonstrated the particle nature of electrons. In this illustration, the profile of the cross-shaped target is projected against the tube face at right by a beam of electrons.[1] |
|
| Composition | Elementary particle[2] |
|---|---|
| Statistics | Fermionic |
| Generation | First |
| Interactions | Gravity, Electromagnetic, Weak |
| Symbol | e−, β− |
| Antiparticle | Positron (also called antielectron) |
| Theorized | Richard Laming (1838–1851),[3] G. Johnstone Stoney (1874) and others.[4][5] |
| Discovered | J. J. Thomson (1897)[6] |
| Mass | 9.10938291(40)×10−31 kg[7] 5.4857990946(22)×10−4 u[7] [1,822.8884845(14)]−1 u[note 1] 0.510998928(11) MeV/c2[7] |
| Electric charge | −1 e[note 2] −1.602176565(35)×10−19 C[7] −4.80320451(10)×10−10 esu |
| Magnetic moment | −1.00115965218076(27) μB[7] |
| Spin | 1⁄2 |
The electron (symbol: e−) is a subatomic particle with a negative elementary electric charge.[8]
An electron has no known components or substructure. It is generally thought to be an elementary particle.[2] An electron has a mass that is approximately 1/1836 that of the proton.[9] The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.
Electrons, which belong to the first generation of the lepton particle family,[10] participate in gravitational, electromagnetic and weak interactions.[11] Like all matter, they have quantum mechanical properties of both particles and waves, so they can collide with other particles and can be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[10]
The concept of an indivisible quantity of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopherRichard Laming;[4] the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.[6][12][13]
In many physical phenomena, such as electricity, magnetism, and thermal conductivity, electrons play an essential role. An electron in motion relative to an observer generates a magnetic field, and will be deflected by external magnetic fields. When an electron is accelerated, it can absorb or radiate energy in the form of photons. Electrons, together with atomic nuclei made of protons and neutrons, make up atoms. However, electrons contribute less than 0.06% to an atom's total mass. The attractive Coulomb force between an electron and a proton causes electrons to be bound into atoms. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[14]
Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. Electrons may be destroyed through annihilation with positrons, and may be absorbed during nucleosynthesis in stars. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including inelectronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Contents |
History
The ancient Greeks noticed that amber attracted small objects when rubbed with fur. Along with lightning, this phenomenon is one of humanity's earliest recorded experiences with electricity.[15] In his 1600 treatise De Magnete, the English scientist William Gilbert coined the New Latin term electricus, to refer to this property of attracting small objects after being rubbed.[16] Both electric and electricity are derived from the Latin ēlectrum(also the root of the alloy of the same name), which came from the Greek word for amber, ήλεκτρον (ēlektron).
In the early 1700s, Francis Hauksbee and French chemist C. F. du Fay independently discovered what they believed to be two kinds of frictional electricity; one generated from rubbing glass, the other from rubbing resin. From this, Du Fay theorized that electricity consists of two electrical fluids, "vitreous" and "resinous", that are separated by friction and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought of the charge carrier as being positive, but he did not correctly identify which situation was a surplus of the charge carrier, and which situation was a deficit.[19]
Between 1838 and 1851, British natural philosopher Richard Laming developed the idea that an atom is composed of a core of matter surrounded by subatomic particles that had unit electric charges.[3] Beginning in 1846, German physicist William Weber theorized that electricity was composed of positively and negatively charged fluids, and their interaction was governed by the inverse square law. After studying the phenomenon of electrolysisin 1874, Irish physicist George Johnstone Stoney suggested that there existed a "single definite quantity of electricity", the charge of a monovalent ion. He was able to estimate the value of this elementary charge e by means ofFaraday's laws of electrolysis.[20] However, Stoney believed these charges were permanently attached to atoms and could not be removed. In 1881, German physicist Hermann von Helmholtz argued that both positive and negative charges were divided into elementary parts, each of which "behaves like atoms of electricity".[4]
In 1894, Stoney coined the term electron to describe these elementary charges, saying, "... an estimate was made of the actual amount of this most remarkable fundamental unit of electricity, for which I have since ventured to suggest the name electron".[21] The word electron is a combination of the word electric(icity) and the Greek suffix "tron", meaning roughly 'the means by which it is done'. The suffix -on which is now used to designate other subatomic particles, such as a proton or neutron, is in turn derived from electron.[22][23]
Discovery
The German physicist Johann Wilhelm Hittorf undertook the study of electrical conductivity in rarefied gases. In 1869, he discovered a glow emitted from the cathode that increased in size with decrease in gas pressure. In 1876, the German physicist Eugen Goldstein showed that the rays from this glow cast a shadow, and he dubbed the rays cathode rays.[25]During the 1870s, the English chemist and physicist Sir William Crookes developed the first cathode ray tube to have a high vacuum inside.[26] He then showed that the luminescence rays appearing within the tube carried energy and moved from the cathode to the anode. Furthermore, by applying a magnetic field, he was able to deflect the rays, thereby demonstrating that the beam behaved as though it were negatively charged.[27][28] In 1879, he proposed that these properties could be explained by what he termed 'radiant matter'. He suggested that this was a fourth state of matter, consisting of negatively charged molecules that were being projected with high velocity from the cathode.[29]
The German-born British physicist Arthur Schuster expanded upon Crookes' experiments by placing metal plates parallel to the cathode rays and applying an electric potentialbetween the plates. The field deflected the rays toward the positively charged plate, providing further evidence that the rays carried negative charge. By measuring the amount of deflection for a given level of current, in 1890 Schuster was able to estimate the charge-to-mass ratio of the ray components. However, this produced a value that was more than a thousand times greater than what was expected, so little credence was given to his calculations at the time.[27][30]
In 1892 Hendrik Antoon Lorentz suggested the idea that the mass of these particles (electrons) could be a consequence of their electric charge.[31]
In 1896, the British physicist J. J. Thomson, with his colleagues John S. Townsend and H. A. Wilson,[12] performed experiments indicating that cathode rays really were unique particles, rather than waves, atoms or molecules as was believed earlier.[6] Thomson made good estimates of both the charge e and the mass m, finding that cathode ray particles, which he called "corpuscles," had perhaps one thousandth of the mass of the least massive ion known: hydrogen.[6][13] He showed that their charge to mass ratio, e/m, was independent of cathode material. He further showed that the negatively charged particles produced by radioactive materials, by heated materials and by illuminated materials were universal.[6][32] The name electron was again proposed for these particles by the Irish physicist George F. Fitzgerald, and the name has since gained universal acceptance.[27]



