Proton
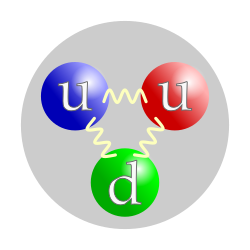 The quark structure of the proton. (The color assignment of individual quarks is not important, only that all three colors are present.) |
|
| Classification | Baryon |
|---|---|
| Composition | 2 up quarks, 1 down quark |
| Statistics | Fermionic |
| Interactions | Gravity, Electromagnetic, Weak,Strong |
| Symbol | p, p+, N+ |
| Antiparticle | Antiproton |
| Theorized | William Prout (1815) |
| Discovered | Ernest Rutherford (1917–1919, named by him, 1920) |
| Mass |
1.672621777(74)×10−27 kg[1] |
| Mean lifetime | >2.1×1029 years (stable) |
| Electric charge | +1 e 1.602176565(35)×10−19 C[1] |
| Charge radius | 0.8775(51) fm[1] |
| Electric dipole moment | <5.4×10−24 e·cm |
| Electric polarizability | 1.20(6)×10−3 fm3 |
| Magnetic moment |
1.410606743(33)×10−26 J·T−1[1] |
| Magnetic polarizability | 1.9(5)×10−4 fm3 |
| Spin | 1⁄2 |
| Isospin | 1⁄2 |
| Parity | +1 |
| Condensed | I(JP) = 1⁄2(1⁄2+) |
The proton is a subatomic particle with the symbol p or p+ and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleusof each atom. The number of protons in each atom is its atomic number. The name proton was given to the hydrogen nucleus by Ernest Rutherford in 1920, because in previous years he had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by collision, and was thus a candidate to be a fundamental particle and building block of nitrogen, and all other heavier atomic nuclei.
In the modern Standard Model of particle physics, the proton is a hadron, composed of quarks. Prior to that model becoming a consensus in the physics community, the proton was considered a fundamental particle. In the modern view, a proton is composed of three valence quarks: two up quarks and one down quark. The rest masses of the quarks are thought to contribute only about 1% of the proton's mass. The remainder of the proton mass is due to the kinetic energyof the quarks and to the energy of the gluon fields that bind the quarks together.
Because the proton is not a fundamental particle, it possesses a physical size—although this is not perfectly well-defined since the surface of a proton is somewhat fuzzy, due to being defined by the influence of forces that do not come to an abrupt end. The proton is about 1.6–1.7 fm in diameter.[2]
The free proton (a proton not bound to nucleons or electrons) is a stable particle that has not been observed to break down spontaneously to other particles. Free protons are found naturally in a number of situations in which energies or temperatures are high enough to separate them from electrons, for which they have some affinity. Free protons exist in plasmas in which temperatures are too high to allow them to combine with electrons. Free protons of high energy and velocity make up 90% of cosmic rays, which propagate in vacuum for interstellar distances. Free protons are emitted directly from atomic nuclei in some rare types ofradioactive decay. Protons also result (along with electrons and antineutrinos) from the radioactive decay of free neutrons, which are unstable.
At sufficiently low temperatures, free protons will bind to electrons. However, the character of such bound protons does not change, and they remain protons. A fast proton moving through matter will slow by interactions with electrons and nuclei, until it is captured by the electron cloud of an atom. The result is a protonated atom, which is a chemical compound of hydrogen. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical. Such "free hydrogen atoms" tend to react chemically with many other types of atoms at sufficiently low energies. When free hydrogen atoms react with each other, they form neutral hydrogen molecules (H2), which are the most common molecular component of molecular clouds in interstellar space. Such molecules of hydrogen on Earth may then serve (among many other uses) as a convenient source of protons for accelerators (as used in proton therapy) and other hadron particle physics experiments that require protons to accelerate, with the most powerful and noted example being the large hadron collider.
Contents |
Description
Protons are spin-½ fermions and are composed of three valence quarks,[3] making them baryons (a sub-type of hadrons). The two up quarks and one down quark of the proton are held together by the strong force, mediated by gluons.[2] A modern perspective has the proton composed of the valence quarks (up, up, down), the gluons, and transitory pairs of sea quarks. The proton has an approximately exponentially decaying positive charge distribution with a mean squareradius of about 0.8 fm.[4]
Protons and neutrons are both nucleons, which may be bound by the nuclear force into atomic nuclei. The nucleus of the most common isotope of the hydrogen atom (with the chemical symbol "H") is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium and tritium contain one proton bound to one and two neutrons, respectively. All other types of atoms are composed of two or more protons and various numbers of neutrons.
Stability
The spontaneous decay of free protons has never been observed, and the proton is therefore considered a stable particle. However, some grand unified theories of particle physics predict that proton decay should take place with lifetimes of the order of 1036 years, and experimental searches have established lower bounds on the mean lifetime of the proton for various assumed decay products.
Experiments at the Super-Kamiokande detector in Japan gave lower limits for proton mean lifetime of 6.6×1033 years for decay to an antimuon and a neutral pion, and 8.2×1033 years for decay to a positron and a neutral pion.[5] Another experiment at the Sudbury Neutrino Observatory in Canada searched for gamma rays resulting from residual nuclei resulting from the decay of a proton from oxygen-16. This experiment was designed to detect decay to any product, and established a lower limit to the proton lifetime of 2.1×1029 years.[6]
However, protons are known to transform into neutrons through the process of electron capture (also called inverse beta decay). For free protons, this process does not occur spontaneously but only when energy is supplied. The equation is:
The process is reversible; neutrons can convert back to protons through beta decay, a common form of radioactive decay. In fact, a free neutron decays this way, with a mean lifetime of about 15 minutes.
Quarks and the mass of the proton
In quantum chromodynamics, the modern theory of the nuclear force, most of the mass of the proton and the neutron is explained by special relativity. The mass of the proton is about 80-100 times greater than the sum of the rest masses of the quarks that make it up, while the gluons have zero rest mass. The extra energy of the quarks and gluons in a region within a proton, as compared to the rest energy of the quarks alone in the QCD vacuum, accounts for almost 99% of the mass. The rest mass of the proton is, thus, the invariant mass of the system of moving quarks and gluons that make up the particle, and, in such systems, even the energy of massless particles isstill measured as part of the rest mass of the system.
Two terms are used in referring to the mass of the quarks that make up protons: Current quark mass refers to the mass of a quark by itself, while constituent quark mass refers to the current quark mass plus the mass of thegluon particle field surrounding the quark.[7] These masses typically have very different values. As noted, most of a proton's mass comes from the gluons that bind the constituent quarks together, rather than from the quarks themselves. While gluons are inherently massless, they possess energy— to be more specific, quantum chromodynamics binding energy (QCBE)—and it is this that contributes so greatly to the overall mass of the proton (seemass in special relativity). A proton has a mass of approximately 938 MeV/c2, of which the rest mass of its three valence quarks contributes only about 11 MeV/c2; much of the remainder can be attributed to the gluons'QCBE.[8]
The internal dynamics of the proton are complicated, because they are determined by the quarks' exchanging gluons, and interacting with various vacuum condensates. Lattice QCD provides a way of calculating the mass of the proton directly from the theory to any accuracy, in principle. The most recent calculations[9][10] claim that the mass is determined to better than 4% accuracy, even to 1% accuracy (see Figure S5 in Dürr et al.[10]). These claims are still controversial, because the calculations cannot yet be done with quarks as light as they are in the real world. This means that the predictions are found by a process of extrapolation, which can introduce systematic errors.[11] It is hard to tell whether these errors are controlled properly, because the quantities that are compared to experiment are the masses of the hadrons, which are known in advance.
These recent calculations are performed by massive supercomputers, and, as noted by Boffi and Pasquini: "a detailed description of the nucleon structure is still missing because ... long-distance behavior requires a nonperturbative and/or numerical treatment..."[12] More conceptual approaches to the structure of the proton are: the topological soliton approach originally due to Tony Skyrme and the more accurate AdS/QCD approachthat extends it to include a string theory of gluons, various QCD-inspired models like the bag model and the constituent quark model, which were popular in the 1980s, and the SVZ sum rules, which allow for rough approximate mass calculations. These methods do not have the same accuracy as the more brute-force lattice QCD methods, at least not yet.
Charge radius
The internationally-accepted value of the proton's charge radius is 0.8768 fm (see orders of magnitude for comparison to other sizes). This value is based on measurements involving a proton and an electron.
However, since 5 July 2010, an international research team has been able to make measurements involving an exotic atom made of a proton and a negatively-charged muon. After a long and careful analysis of those measurements, the team concluded that the root-mean-square charge radius of a proton is "0.84184(67) fm, which differs by 5.0 standard deviations from the CODATA value of 0.8768(69) fm".[13] In January 2013 an updated value for the charge radius of proton—0.84087(39) fm was published. The precision was improved by 1.7 times, but the difference with CODATA value persisted at 7σ significance.[14]
The international research team that obtained this result at the Paul Scherrer Institut (PSI) in Villigen (Switzerland) includes scientists from the Max Planck Institute of Quantum Optics (MPQ) in Garching, the Ludwig-Maximilians-Universität (LMU) Munich and the Institut für Strahlwerkzeuge (IFWS) of the Universität Stuttgart (both from Germany), and the University of Coimbra, Portugal.[15][16] They are now attempting to explain the discrepancy, and re-examining the results of both previous high-precision measurements and complicated calculations. If no errors are found in the measurements or calculations, it could be necessary to re-examine the world's most precise and best-tested fundamental theory: quantum electrodynamics.[17]
Interaction of free protons with ordinary matter
Although protons have affinity for oppositely-charged electrons, free protons must lose sufficient velocity (and kinetic energy) in order to become closely associated and bound to electrons, since this is a relatively low-energy interaction. High energy protons, in traversing ordinary matter, lose energy by collisions with atomic nuclei, and by ionization of atoms (removing electrons) when until they are slowed sufficiently to be captured by the electron cloud in a normal atom.
However, in such an association with an electron, the character of the bound proton is not changed, and it remains a proton. The attraction of low-energy free protons to any electrons present in normal matter (such as the electrons in normal atoms) causes free protons to stop and to form a new chemical bond with an atom. Such a bond happens at any sufficiently "cold" temperature (i.e., comparable to temperatures at the surface of the Sun) and with any type of atom. Thus, in interaction with any type of normal (non-plasma) matter, low-velocity free protons are attracted to electrons in any atom or molecule with which they come in contact, causing the proton and molecule to combine. Such molecules are then said to be "protonated", and chemically they often,
Electron
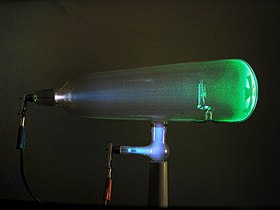 Experiments with a Crookes tube first demonstrated the particle nature of electrons. In this illustration, the profile of the cross-shaped target is projected against the tube face at right by a beam of electrons.[1] |
|
| Composition | Elementary particle[2] |
|---|---|
| Statistics | Fermionic |
| Generation | First |
| Interactions | Gravity, Electromagnetic, Weak |
| Symbol | e−, β− |
| Antiparticle | Positron (also called antielectron) |
| Theorized | Richard Laming (1838–1851),[3] G. Johnstone Stoney (1874) and others.[4][5] |
| Discovered | J. J. Thomson (1897)[6] |
| Mass | 9.10938291(40)×10−31 kg[7] 5.4857990946(22)×10−4 u[7] [1,822.8884845(14)]−1 u[note 1] 0.510998928(11) MeV/c2[7] |
| Electric charge | −1 e[note 2] −1.602176565(35)×10−19 C[7] −4.80320451(10)×10−10 esu |
| Magnetic moment | −1.00115965218076(27) μB[7] |
| Spin | 1⁄2 |
The electron (symbol: e−) is a subatomic particle with a negative elementary electric charge.[8]
An electron has no known components or substructure. It is generally thought to be an elementary particle.[2] An electron has a mass that is approximately 1/1836 that of the proton.[9] The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.
Electrons, which belong to the first generation of the lepton particle family,[10] participate in gravitational, electromagnetic and weak interactions.[11] Like all matter, they have quantum mechanical properties of both particles and waves, so they can collide with other particles and can be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.[10]
The concept of an indivisible quantity of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopherRichard Laming;[4] the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.[6][12][13]
In many physical phenomena, such as electricity, magnetism, and thermal conductivity, electrons play an essential role. An electron in motion relative to an observer generates a magnetic field, and will be deflected by external magnetic fields. When an electron is accelerated, it can absorb or radiate energy in the form of photons. Electrons, together with atomic nuclei made of protons and neutrons, make up atoms. However, electrons contribute less than 0.06% to an atom's total mass. The attractive Coulomb force between an electron and a proton causes electrons to be bound into atoms. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.[14]
Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. Electrons may be destroyed through annihilation with positrons, and may be absorbed during nucleosynthesis in stars. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including inelectronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Contents |
History
The ancient Greeks noticed that amber attracted small objects when rubbed with fur. Along with lightning, this phenomenon is one of humanity's earliest recorded experiences with electricity.[15] In his 1600 treatise De Magnete, the English scientist William Gilbert coined the New Latin term electricus, to refer to this property of attracting small objects after being rubbed.[16] Both electric and electricity are derived from the Latin ēlectrum(also the root of the alloy of the same name), which came from the Greek word for amber, ήλεκτρον (ēlektron).
In the early 1700s, Francis Hauksbee and French chemist C. F. du Fay independently discovered what they believed to be two kinds of frictional electricity; one generated from rubbing glass, the other from rubbing resin. From this, Du Fay theorized that electricity consists of two electrical fluids, "vitreous" and "resinous", that are separated by friction and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought of the charge carrier as being positive, but he did not correctly identify which situation was a surplus of the charge carrier, and which situation was a deficit.[19]
Between 1838 and 1851, British natural philosopher Richard Laming developed the idea that an atom is composed of a core of matter surrounded by subatomic particles that had unit electric charges.[3] Beginning in 1846, German physicist William Weber theorized that electricity was composed of positively and negatively charged fluids, and their interaction was governed by the inverse square law. After studying the phenomenon of electrolysisin 1874, Irish physicist George Johnstone Stoney suggested that there existed a "single definite quantity of electricity", the charge of a monovalent ion. He was able to estimate the value of this elementary charge e by means ofFaraday's laws of electrolysis.[20] However, Stoney believed these charges were permanently attached to atoms and could not be removed. In 1881, German physicist Hermann von Helmholtz argued that both positive and negative charges were divided into elementary parts, each of which "behaves like atoms of electricity".[4]
In 1894, Stoney coined the term electron to describe these elementary charges, saying, "... an estimate was made of the actual amount of this most remarkable fundamental unit of electricity, for which I have since ventured to suggest the name electron".[21] The word electron is a combination of the word electric(icity) and the Greek suffix "tron", meaning roughly 'the means by which it is done'. The suffix -on which is now used to designate other subatomic particles, such as a proton or neutron, is in turn derived from electron.[22][23]
Discovery
The German physicist Johann Wilhelm Hittorf undertook the study of electrical conductivity in rarefied gases. In 1869, he discovered a glow emitted from the cathode that increased in size with decrease in gas pressure. In 1876, the German physicist Eugen Goldstein showed that the rays from this glow cast a shadow, and he dubbed the rays cathode rays.[25]During the 1870s, the English chemist and physicist Sir William Crookes developed the first cathode ray tube to have a high vacuum inside.[26] He then showed that the luminescence rays appearing within the tube carried energy and moved from the cathode to the anode. Furthermore, by applying a magnetic field, he was able to deflect the rays, thereby demonstrating that the beam behaved as though it were negatively charged.[27][28] In 1879, he proposed that these properties could be explained by what he termed 'radiant matter'. He suggested that this was a fourth state of matter, consisting of negatively charged molecules that were being projected with high velocity from the cathode.[29]
The German-born British physicist Arthur Schuster expanded upon Crookes' experiments by placing metal plates parallel to the cathode rays and applying an electric potentialbetween the plates. The field deflected the rays toward the positively charged plate, providing further evidence that the rays carried negative charge. By measuring the amount of deflection for a given level of current, in 1890 Schuster was able to estimate the charge-to-mass ratio of the ray components. However, this produced a value that was more than a thousand times greater than what was expected, so little credence was given to his calculations at the time.[27][30]
In 1892 Hendrik Antoon Lorentz suggested the idea that the mass of these particles (electrons) could be a consequence of their electric charge.[31]
In 1896, the British physicist J. J. Thomson, with his colleagues John S. Townsend and H. A. Wilson,[12] performed experiments indicating that cathode rays really were unique particles, rather than waves, atoms or molecules as was believed earlier.[6] Thomson made good estimates of both the charge e and the mass m, finding that cathode ray particles, which he called "corpuscles," had perhaps one thousandth of the mass of the least massive ion known: hydrogen.[6][13] He showed that their charge to mass ratio, e/m, was independent of cathode material. He further showed that the negatively charged particles produced by radioactive materials, by heated materials and by illuminated materials were universal.[6][32] The name electron was again proposed for these particles by the Irish physicist George F. Fitzgerald, and the name has since gained universal acceptance.[27]